ABOUT THE APPLICATION
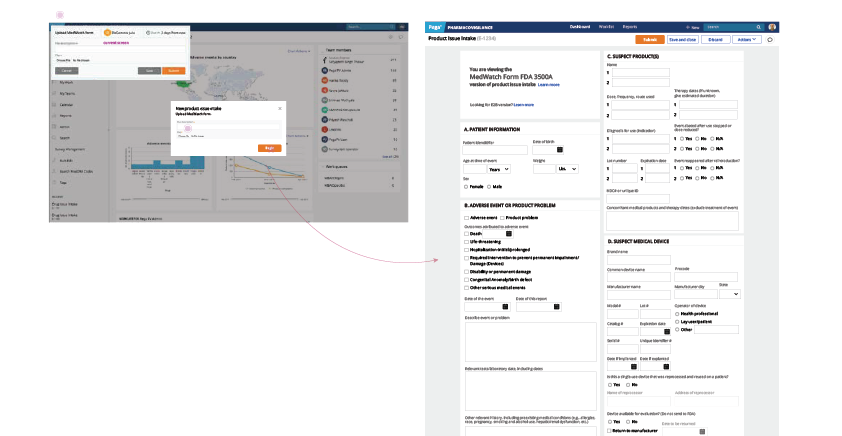
Pega Pharmacovigilance (PV) is an end-to-end safety solution which allows life sciences companies to collect, analyze, and distribute safety information to their business affiliates or to regulatory agencies like FDA, EMEA, etc. In other words, when someone has a drug/any medicinal product and something goes wrong with the product, the user (you, in this case) must capture the event (called an adverse event or product complaint case) using this application Ultimately, pharmacovigilance is concerned with identifying the haz- ards associated with pharmaceutical products and with minimizing the risk of any harm that may come to patients. Companies must con- duct a comprehensive drug safety and pharmacovigilance audit to assess their compliance with worldwide laws, regulations, and guid- ance.
CHALLENGES
- Pharmacovigilance application was overwhelmed with the amount of information and data in the screens.
- On most of the screens it was difficult for the user to figure out what they were supposed.
- Lots of unnecessary fields without proper helper text/guidelines to the user.
- Logical grouping of the information is not appropriate.
- Naming conventions are not consistent
- Visual ques for mandatory information is missed
- To maintain consistency while user interacting with different elements on the screens
USABILITY TESTING
The goals of the Pharmacovigilance application usability tests were to analyze the current Phar- macovigilance application experience and to learn where there may be room for improvement. Two Pega employees varying in technical/Pega experience participated in this study. Both participants found the experience to be quite unintuitive, and both stated that they found the screens to be overwhelming.
USABILITY RECOMMENDATIONS
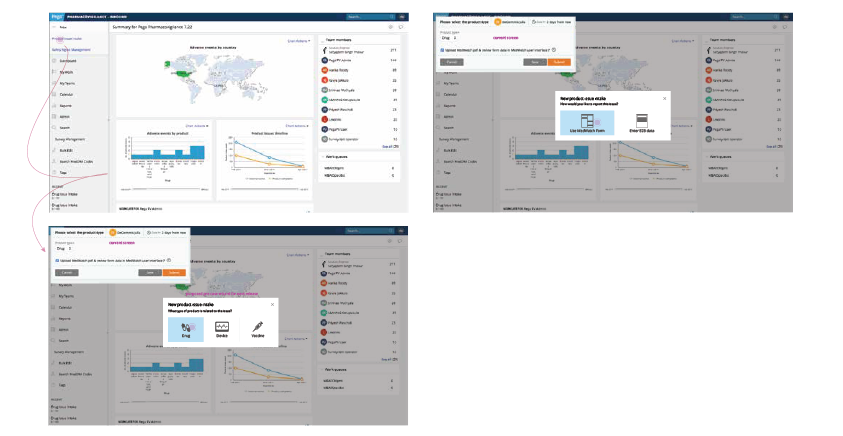
- Since the main action for the user is to select a product type, remove unnecessary infor- mation and make the product type selection more obvious.
- Remove unnecessary fields and include some helper text regarding the receipt.
- Include the ability to import information to this screen. Make the “Add reporter informa- tion” section look mandatory and change the button labels to “Search for existing report- er” and “Create new reporter”.
- Automatically create an entry because the user entered in product information earlier in this process.
PERSONA’S
Pega PV Intake user

Dan Underwood
Product issue intake user
Dan Captures product issue and sends the case for review to medical review specialist
Medical review specialist

Zoe Wilson
Medical review specialist
Zoe will reviews the product issue intake case created by intake user and updates the case accordingly
Quality control reviewer

Megan Taylor
Quality control reviewer
Megan review updates made by MR specialist and updates the case accordingly
Outbound safety admin

Adam Blake
Outbound safety admin
Adam generates reports to be sent to FDA. Maintains the adverse event report generation rules and also reviews the generated reports
Country/specific admin

Shou Umi
Country/specific admin
Show Umi Review the report generat- ed for a speciic destination
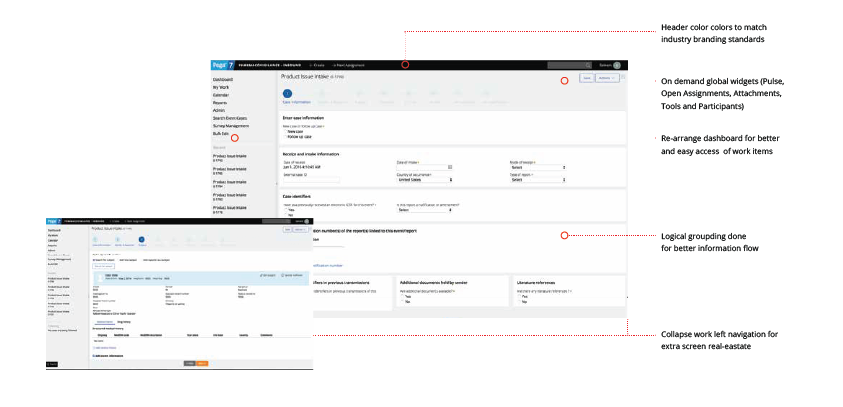
NEW APPLICATION SCREENS